
The Future of Lean Lab Implementations
As laboratories become more and more digitalized and lab systems are increasingly efficiency-driven, many people may begin by asking whether Lean methodologies are still even relevant. In this comprehensive article, I hope to make a case for why they are, as well as to demonstrate how digitalization actually facilitates and enhances the implementation of lean labs. This article provides an overview of the topics covered in our recent #binocstalks webinar delivered by Greg Heaslip. You can view a recording of the complete webinar here.
Before joining Bluecrux as Binocs Sales Director, Greg Heaslip worked first as a Principal Consultant specializing in Lean implementations for BSM and later founded Plan Domino, a software company that applied his extensive knowledge of lean principles to the digital lab scheduling sector. Bluecrux acquired Plan Domino in 2021.
Lean concepts
Why implement lean lab methodologies?
At its core, the intent of Lean is “the complete and thorough elimination of wasteful practices”, which ultimately means seeking to make gains across the organization by reducing and removing inefficiencies wherever possible. Modern pharmaceutical labs can achieve these gains in a number of key areas, including:
- Productivity, ensuring that workload is leveled and distributed evenly, fairly, and with minimal disruption to delivery schedules;
- Morale, ensuring that the workforce has a clarity of purpose and priorities, leading to greater retention of highly-trained staff;
- Environmental, with greener processes that consume less water, fewer chemicals and require fewer test runs, equipment usage, etc.;
- Communication, facilitating clear channels both internally and externally;
- Quality, both in terms of product but also timely service delivery and improvements to right-first-time test and release.
The ‘7/8 wastes’
To help identify potential gains, Lean systems often refer to the “7 wastes”. While these may superficially appear to be more relevant to the materials and product manufacturing sector in which they were developed, they are nevertheless topics worth exploring at different stages in the laboratory supply chain. They represent opportunities to increase efficiency by reducing or eliminating waste from:
- product or service defects that fail to meet customer expectations
- overproduction, causing supply to exceed demand
- time spent waiting for the next process step
- time spent in unnecessary transportation of products and materials
- unprocessed materials expiring while still in inventory
- the unnecessary movement of people
- extra-processing of the product beyond what is necessary to meet the quality standards
Additionally, an 8th waste is now commonly also included that is perhaps most relevant to the pharma industry: waste from unused talent, as people’s skills and knowledge are consistently underutilized. This is especially pertinent when considering the potential impact of capacity planning as a critical pillar in House of Lean Lab.
The House of Lean Labs
There are many variations on the “House of Lean” concept, in which the overall lean enterprise is constructed in levels, up from solid foundations towards the ultimate goal of optimized lean performance. Below is our version:
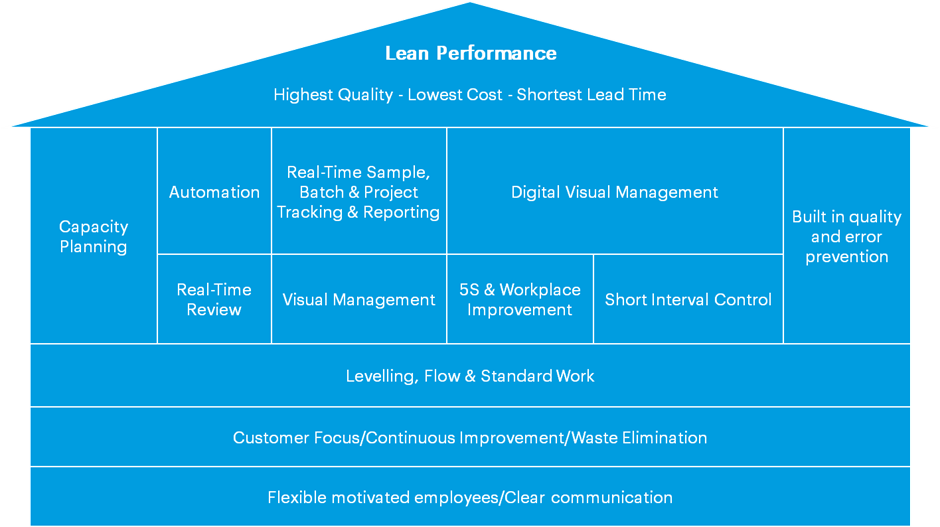
When implementing lean methodologies in a modern lab, it is generally assumed that the bottom two levels of the house – having flexible and motivated employees and engaging in customer-focused activities, continuous improvement practices and eliminating physical waste – will already be established as a solid foundation.
The next critical step is to ensure that leveling, flow, and standard work processes are in place; these will give labs the tools and procedures required to develop an effective lean laboratory. Standard work, in particular, allows planners to build the crucial pillar of dedicated capacity planning, while clear channels of communication, feedback, and monitoring allow not only the effective implementation of leveling and flow practices but also supports the creation of channels to facilitate error prevention and enhance quality.
Leveling, Flow & Standard Work
Volatility
Workload volatility basically describes the significant oscillations in the volume of requests that arrive in a typical lab, cycling between high demand (that can outstrip capacity and cause analyses to run late) and low demand (that can result in poor productivity). Calculating the average workload over a period (daily, weekly, monthly or even quarterly) produces a “leveled demand rate”, effectively the amount of work (and thus the resource level) that would be expected if demand were perfectly stable.

As a result, labs tend to exercise caution and plan resource availability to a level that can meet the demand peaks with small but manageable degrees of late delivery. This necessarily means provisioning above what would be required to meet the leveled demand rate; the greater the volatility, the greater the divergence between optimal resourcing and what is needed to minimize late delivery.
This gap means that there will be periods when a laboratory has excess resources and other periods when they require additional capacity; however, it also represents an opportunity: by implementing a system that allows effective and dynamic leveling and flow, labs can more efficiently meet demand (typically by increasing capacity as required for peak periods).
Leveling
Leveling exercises involve identifying strategies to help shift resource capacity closer towards the leveled demand rate (usually calculated from historical data). Traditional lean systems generally implement three main leveling strategies:
- Queueing tasks as they arrive and managing them accordingly so that workload is distributed more evenly for individuals across the day/week/month;
- Mixing workstreams (for instance, stability and release teams in a QC lab) such that combining multiple volatile workflows can result in a more stable workload distribution across the combined team;
- Linking to consumption (for instance, in a raw materials lab), which represents a far less volatile demand cycle than linking to purchasing.
Flow
The term “flow” refers to the rapid movement of work (projects/samples/batches) through all required activities in the lab, from the first step in the process to full completion, with minimal delay. Work can be queued on arrival; however, once an activity has started, it should be finished as quickly as possible. Flow is achieved by having clearly-defined sequences of activities, formalized via processes such as trains and rhythm wheels, and supported through standard work techniques and role card systems.
Rhythm wheels and trains
Rhythm wheels are typically a one- or two-week cycle of repeating activities, with patterns developed based on historical data. In the below example of a one-week rhythm wheel, a set sequence of tasks will be performed on each day of the week, a pattern that then begins again after 7 days. For instance, Test a is performed on Mondays, Wednesdays and Fridays, while Tests b-e are performed on Tuesdays, and so on.

The rhythm wheel system is highly effective in setting out a fixed program of work but it is, by definition, highly inflexible – if something goes wrong, it can be very difficult to adapt the system to accommodate unexpected changes (which are all-too-common in a busy pharma laboratory). In the above example, it is clear that partial tests or retests can cause unrelated test plans to be postponed, with significant and aggregate consequences for on-time delivery.
This means that allowances must be built-into rhythm wheels to accommodate the late or incomplete arrival of testing materials, unexpected analyst absences, equipment unavailability, delays, postponements, and other typical features of dynamic work. Such allowances necessarily reduce efficiency. Furthermore, the static nature of rhythm wheels can often cause them to fail during holiday seasons when the prescriptive and inflexible system cannot readily adapt to changing work patterns without significant additional ad hoc input from planners.
Greater flexibility is possible using a train system, which is better-suited to high-volatility workloads. In the train system, a specific sequence of testing events (e.g. preparation à testing à write-up à review) is defined in advance, with particular individuals and equipment assigned to each step. Samples that arrive in the lab are allocated to the relevant train depending on the required method. The train “leaves the station” (i.e. the sequence of analysis steps begins) either when the must start date is reached or when the train is at full capacity (usually determined by the capacity of the equipment used for the analysis).
Whether using rhythm wheels or trains, the development of standard work is crucial to successful implementation.
Standard work
Standard work is essentially a way of formalizing and standardizing work by defining the ideal procedures, sequences, and timings required to complete a specific test method or other processes calculated based on historical data. In theory, with access to the same materials, equipment, and training, any analyst should be able to complete the same test following the same pattern and in the same timeframe, irrespective of their location.

As per the above example, standard work can be outlined in a role card that details each step required for a test to be completed, including the method sample capacity, expected equipment usage time, and individual responsibilities. Existing users of the Binocs system will recognize this as being very similar to the design of “services”.
Planning boards
Once a lab has completed the preparatory work to establish new standardized systems and processes, the next logical step is to create a planning board to aid in managing all the different moving parts. Below is an example of the last manual planning board that Greg worked on before moving toward digital solutions:

In this example, as samples arrive in the lab, a new card is created (representing either the individual sample or trains/batches of samples) that is then added to the bottom of the queue on the left hand side. As the “must start” date approaches, the card ① moves up the queue until it is time to begin the analysis; it is then ② allocated to and analyst and equipment on a given day of the coming week as required to complete the testing on time; once the test is completed, the card can be ③ moved across for review and eventual release.
This process is highly visual and allows any member of lab staff to ascertain, at a glance, which samples are queued, which are in processing and by whom, and which are ready for the next stage in the process towards release.
“Quick wins” for lean labs
There are a variety of quick wins that can be readily and rapidly implemented in the laboratory to introduce lean practices with immediate benefit but without requiring significant financial expenditure or sourcing external expertise.
Daily stand-up/huddle
Most labs have already implemented a daily stand-up meeting in their teams, and those that haven’t should seriously consider doing so as very easy “quick win”.
These huddles should be rapid-fire meetings involving the core lab team and with a fixed agenda focused on activities planned for yesterday, today and tomorrow, outlining the high-level status of different workstreams and highlighting issues that have arisen since the last meeting. Generally, the lab’s planning board is the ideal focal point for such meetings as all of the pertinent information should be readily available. Crucially, these huddles should last no longer than 10 minutes, with lengthy discussions moved into separate meetings involving only the relevant team members.

Many labs who use this approach will state that their daily stand-ups last no longer than 10 minutes but, in practice, they often suffer from significant time creep that reduces the availability of attendees to do perform their tasks. Effective time management is critical, and leadership of the huddle should be rotated regularly to avoid stagnation and ensure that everyone has an opportunity to guide the process.
5/6S
5S methodologies represent a great opportunity for quick wins in the lab. They function both to unify and orient the team towards lean processes and to better organize the physical workplace, such that all equipment and workstations are identifiable and accessible.
There are a lot of detailed online resources to aid in implementing these practices, a few of which are listed below:
It is worth noting, however, that 5/6S programs must be fit-for-purpose. It is possible to implement a methodology in a way that actually results in greater complexity, rather than greater clarity. As such, sufficient time must be taken to assess the utility and practicality of maintaining a system that merely shifts inefficiencies from one area of work to another, rather than eliminating them. As in all things, such processes should be subject to rigorous and regular feedback and refinement.
Visual management
Visual management methodologies are another quick win that labs can implement with relative ease. These can lead to embedded and sustained lean processes while allowing any team member to visualize the lab’s short interval (day-to-day) performance and facilitating pro-active intervention in response to issues as they arise. This creates a clear, shared understanding of lab performance and operational status, while also supporting continuous improvement activities by providing clear standards.
The fundamental philosophy behind good visual management in the lab is expressed in the following quote:
Anyone should be able to walk (or log) in and understand the current operational status and the lab’s medium term performance, in 5 minutes or less without speaking to anyone.
This standard should be the yardstick against which labs measure the success of their visual management processes.
The processes themselves centre around the display of unambiguous visual signals and controls to convey key information that can be understood quickly by everyone and allow them to direct activities, communicate progress and highlights issues.
Examples of quick win visual management systems include:
- Physical sample trains, where boxes are clearly-delineated for storing batches, all related documentation and relevant information, such as the must start date;
- Batch record prioritization, where physical files are stored in a clearly-labeled fashion that allows anyone to immediately see which tests are due and which are late;
- T-card systems, which can be used to organize samples and actions into visual categories that allow for an at-a-glance status update or backlog overview;
- Visual sample trolleys, where samples are physically placed on a trolley with segregated queue segments for different products and remain on the trolley as it is moved from one test to another on completion. These are especially appropriate for short-duration sequential testing (e.g chemistry labs) and can eliminate waste as the approach requires only one sample bottle per batch (as opposed to one per test day), facilitates all tests on one router and reduces manual paperwork log maintenance.

How has digital changed lean labs?
Digital management systems
The vast majority of labs have already implemented some form of LIMS and/or ELN and these do introduce some clear Lean benefits, especially in terms of reducing the manual workload associated with quality control and back-end administration. However, as already expressed above, the most impactful gains can be made through improved leveling, flow and standard work; this is where digital systems have emerged as game-changers that significantly reduce the complicated and labor-intensive manual processes associated with scheduling and capacity management.
In particular, digital scheduling systems allow for the dynamic calculation of flex time, automated sample campaigning, and the calculation of must start dates and sample prioritization. By enabling end-to-end access, labs can connect directly to their QA and supply chain partners and review testing status via digital visual management processes (see below).
Automation and robotics
Another area in which digital processes have accelerated the potential of Lean labs is automation and robotics. These present an opportunity to replace or repurpose human factors in the lab, for instance through robotic sample processing. However, they also typically come with significant up-front costs, both in terms of financial investment but also the resources required for reorganization and change management. It can therefore be an extremely valuable exercise to first run simulations of the enhancements that such automation might deliver. Using a digital capacity management system like Binocs allows labs to model what-if scenarios of potential capacity increases.
Flexibility in the face of change
One great benefit of digital lean systems compared with paper-based (or even Excel-based) systems is their inherent flexibility and ability to adapt to the ever-present risk of change. Whereas lean methodologies might have previously failed due to unexpected changes to test methods, demand, resource availability, and equipment uptime or from sudden increases in retest rates, dedicated digital systems allow adaptations to be implemented at the touch of a button, with modifications in one part able to cascade throughout the system.
Digital visual management
With the increasing digitization of labs, it is only natural that visual management should also move into the digital sphere. Indeed, the above-described manual planning board is what encouraged Greg to explore digital alternatives – and the benefits are self-evident.
Below is an example of the Binocs digital scheduling board being used in a lab on a large touchscreen display, effectively replacing the manual card-based system with a single source of truth that can be accessed and edited from any location. The principle remains the same: tasks are allocated to individual analysts and instruments (down the left-hand side of the screen) and distributed across the days of the week (along the top); as tasks are completed they are moved to different part of the display and eventually marked as ‘executed’, providing a clear digital paper trail that is easily verified.

Visual management is not simply restricted to data input, however. Another significant benefit of digital management systems for capacity and scheduling, such as Binocs, is the centralization of lab data that facilitates advanced back-end analysis so that results can be visually presented in real time and in a manner that generates meaningful insights.
For instance, knowing the status of all test batches allows such systems to provide clear metrics of a sample’s progress through the lab. Below is an example of the Binocs predictive batch tracker, which (much like the physical batch record prioritization system described above) highlights how many tests will be delivered late and which tests can still be executed on time:

Similar visualizations can also readily display at-a-glance info about any asset that is represented in the digital system. For instance, reports on equipment and team member utilization that allows users to identify exactly where resources are being applied; tracking retest / right first time KPIs; and entire project workflows displayed in interactive Gantt charts.
Conclusion – an additional quick(ish) win
Digital has undoubtedly enhanced the reach and impact of Lean methodologies and, in many cases, entirely replaced older, physical systems. Some things haven’t changed, though, and there remain a variety of ways in which traditional Lean systems continue to be hugely beneficial.
As long as labs still work on physical samples, the manual systems described above can still deliver efficiency gains, such as by implementing physical 5S systems to organize the workspace and physical visual management systems to organize documents and the samples themselves. Likewise, labs should continue to employ tried and tested methods for identifying efficiency gains, such as conducting pareto analyses of services, following continuous improvement frameworks, and giving due consideration to waste elimination.
That said, a good way to significantly enhance a lab’s Lean credentials is to implement a digital scheduling and capacity management system. While, it will require external expertise, a dedicated digital system such as Binocs represents relatively quick win because it can be implemented in under 14 weeks, is highly cost-effective, sits outside QMS (and so is not subject to length validation processes), and is entirely consistent with the typical corporate digital strategy followed by most organizations.
Additionally, unlike scheduling systems that are built as add-ons to existing LIMS platforms, dedicated SaaS solutions such as Binocs are not dependent on other systems, vendors or third-party upgrade schedules, and can readily integrate agnostically with pre-existing digital tools.
Want to learn more about Lean strategies for the modern pharma lab? Why not download our white paper “Faster, better, cheaper: 5 principles to improve lab performance using Lean Lab strategies”
Adam Lester-George
Adam has two decades of experience working in clinical trials, biomedical research, public health, and health economics, with a particular interest in the intersection between technology and life sciences. For 7 years before joining Bluecrux in 2019, Adam was the director of healthcare innovation consultancy “LeLan” and brings a wide range of insights to his role as Content Specialist for Binocs.



